The movement of biological substances plays a crucial role in diagnosis, treatment, and research in medical laboratories, research facilities, and even in everyday healthcare settings. Biological materials which are dangerous goods must be safely transported from the sender (shipper/consignor) to the receiver to prevent exposure or release to protect workers, the public and the environment.
Biological Substances
The primary safety risk when transporting biological materials lies in the potential exposure to undiagnosed diseases that are not on the Category A Infectious substances list. Biological Substances includes:
◾ Human or animal material such as blood, tissue, tissue fluids that are not listed as infectious and pathogens are present.
◾ Clinical Waste that is generated as part of the medical treatment for items that are not identified as infectious.
◾ Patient Specimens that are not part of an exemption. Items that would be Exempt e.g. Blood or Urine tests, that monitor cholesterol, blood sugar levels, prostate-specific antigens (PSA), hormones, drug tests.
◾ Biological Products that are known to contain pathogens that are not packaged and distributed for use by medical professionals.
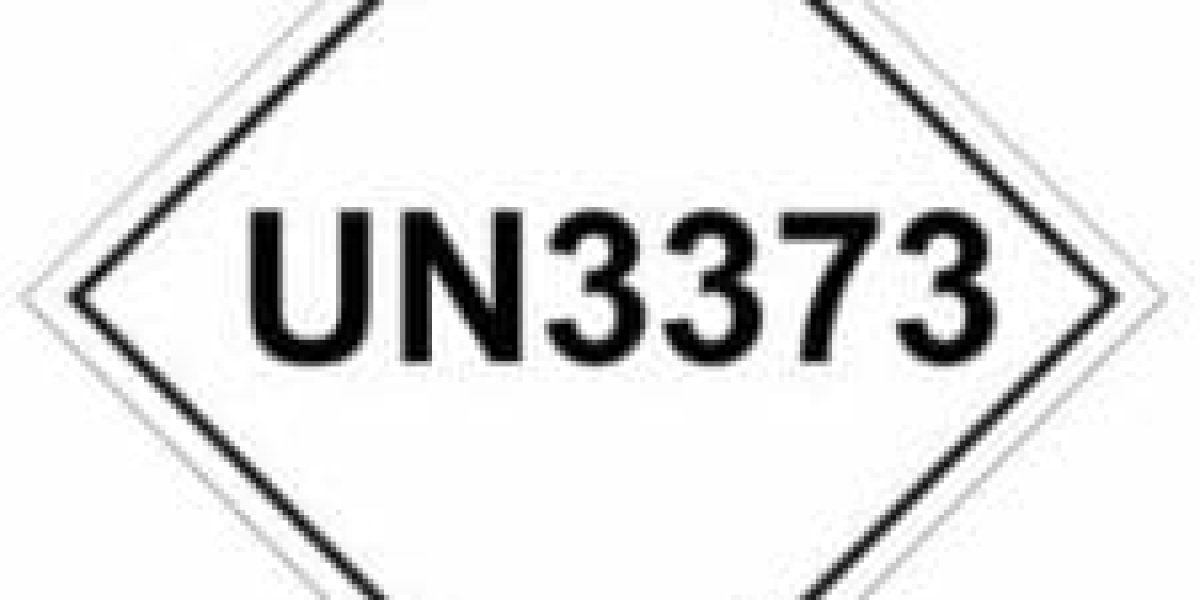
These substances are given the classification ‘UN 3373 Biological Substance Category B’.
More on Class 6 here
So how is the risk minimised?
Robust risk assessment procedures should be put in place to identify potential hazards and develop appropriate control measures. This includes selecting suitable packaging materials, implementing stringent hygiene protocols, and providing personal protective equipment (PPE) to transport personnel where necessary.
Regular training programs are essential to ensure that all individuals involved in the transportation process are aware of safety procedures and best practices. Additionally, ongoing monitoring and compliance audits help to identify any deficiencies and ensure continuous improvement in safety standards.
Occupational Hazards for Transporters
In the UK the transportation of biological substances is exempt from the road (ADR), International Maritime Dangerous Goods Code (IMDG) and Air International Civil Aviation Organization (ICAO) requirements provided it is packed in according with the packaging instructions.
That does not mean transporting biological materials does not present occupational hazards to the personnel involved. Workers responsible for packaging, loading, unloading, and transporting these materials need to be careful.
Clear instructions for the filling and closing of the packages must be provided by the packaging manufacturer and any subsequent distributor of the package. Especially if the person sealing the package, is a patient.
Occasionally items need to be transported with dry ice and nitrogen to keep the item refrigerated or in a frozen state. Both of these items are an asphyxiant so need professional handling.
Training requirements for those involved in the transportation process ensure that they are equipped with the necessary knowledge and skills to handle biological substances safely.
Transporting these substances requires adherence to strict packaging standards, including the use of leak-proof primary receptacles, secondary packaging, and absorbent materials to contain spills.
Packaging standards are stringent to prevent leaks, spills, and contamination during transportation. The packaging must consist of leak-proof primary receptacles, secondary packaging, and absorbent materials to contain spills. Packaging materials should be durable and capable of withstanding the conditions of transport
Documentation Requirements
There are no documentary requirements for the transport of biological substances, but we should always clearly communicate that UN3373 is being transported.
Labeling and Marking
Proper labeling and marking of packages containing biological substances are essential for identification and hazard communication.

Labels must display the appropriate hazard symbols, such as the international biohazard symbol, along with the UN number and proper shipping name. Additionally, packages must bear the name and address of the sender and recipient and an Emergency Contact.
Training and Competency
Personnel involved in the transportation of biological substances must receive adequate training to ensure they understand the risks involved and know how to handle and transport such materials safely. Training programs cover topics such as packaging and transport requirements, emergency procedures, and the use of personal protective equipment (PPE).
Regulatory Authorities
By adhering to these regulations and implementing proper safety measures, shippers can mitigate the risks associated with the transportation of infectious substances and ensure the safe and secure delivery of these materials for medical, research, and diagnostic purposes in the United Kingdom.
It is worth remembering that when sending shipments by air, some countries (states) or Airlines (operators) may have restrictions on quantities or may even refuse to accept packages containing biological substances.
If you require any further advice regarding shipping biological substances, then please contact our team.